
(Clockwise) Scientists made use of XSEDE-allocated supercomputers Stampede2 of the Texas Advanced Computing Center, Comet of San Diego Supercomputer Center; as well as NIH-funded Anton 2 of the Pittsburgh Supercomputing Center and NSF-funded Blue Waters of the National Center for Supercomputing Applications. (Credit: TACC/SDSC/PSC/NCSA)
Using XSEDE supercomputers, scientists have developed for the first time a way to screen drugs through their chemical structures for induced arrhythmias. Death from sudden cardiac arrest causes the most deaths by natural causes in the U.S. estimated at 325,000 per year.
The heart’s bioelectrical system goes haywire during arrest. The malfunction can send heartbeats racing out of control, cutting off blood to the body and brain. This differs from a heart attack, which is caused by a blockage of the heart’s arteries. The leading risk factors for sudden cardiac arrest are a past attack and the presence of disease. Another risk factor is the side effects from medications, which can cause deadly arrhythmias.
Up until the early 2000s, the reason most drugs were removed from the market following FDA approval was cardiotoxicity in the form of deadly arrhythmia. In 2005, the FDA required a separate test for all drugs. It measured the average time between the Q and T waves on an electrocardiogram, a record of the heartbeat. QT prolongation became a red flag for drug cardiotoxicity. But one problem is that some harmless substances, like grapefruit juice, also prolong QT interval, and using it as a proxy for heart arrhythmia could mean the loss of potentially useful and safe drugs”What we set out to do was to try to solve that problem by building a computer-based pipeline for screening,” said Colleen Clancy, a professor in the Department of Physiology and Membrane Biology and the Department of Pharmacology at the UC Davis School of Medicine. Clancy co-authored a study on the computational cardiotoxicity drug screening pipeline in the journal Circulation Research in April 2020.
“The major novelty of the pipeline is that we found a way to connect the atomistic scale to higher level function scales, like protein function, cell function, and in our simulated tissue-level models we can calculate the spatial and temporal gradients of electrical activity in those simulated pieces of tissue,” Clancy said. “That is an approximation of the electrocardiogram that’s measured in the clinic. We can do a direct comparison between the electrocardiogram in the simulated tissue, and electrocardiograms from patients that have taken those drugs.”
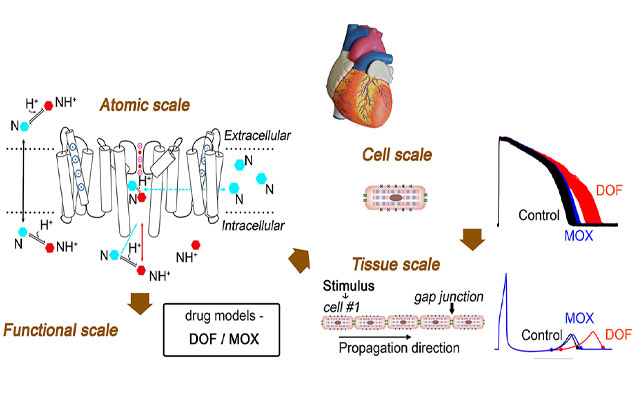
A computational pipeline to screen drugs for cardiotoxicity has been developed with the use of supercomputers. The pipeline connects atomistic scale information to protein, cell, and tissue scales by predicting drug-binding affinities and rates from simulation of hERG ion channel and drug structure interactions and then using these values to model drug effects on the hERG ion channel function and an emergent cardiac electrical activity alteration. (Credit: Yang et al., Circulation Research)
The two drugs chosen in the study both prolonged the QT interval. One of them, dofetilide, is a known proarrhythmic agent. The other, moxifloxacin, has a strong safety profile in healthy humans.
“There’s been no way to distinguish between those two classes,” Clancy said. “That’s what we were able to show in the computational pipeline.” Starting from the chemistry of the drug interactions with a target, the scientists used that information to predict proarrhythmia vulnerability through a machine learning approach based on multi-scale computer simulation data.
Clancy and colleagues chose the hERG (human Ether-à-go-go-Related Gene) potassium channel in the heart as the drug target in the first step of their computational pipeline. The hERG mediates the electrical activity of the heart, and drug companies usually screen for whether a drug blocks it.
The big challenge computationally is the system that we studied is pretty large,” said study co-author Igor Vorobyov, an assistant professor in the Department of Physiology and Membrane Biology and the Department of Pharmacology at the UC Davis School of Medicine. “It’s on the atomistic scale. We have around 130,000 atoms in our system. This includes the hERG protein embedded in the lipid membrane surrounded in a salt aqueous solution in water.”
The calculations involved billions of individual time steps to achieve an all-atom simulation of several microseconds, enough to get detailed information on how the drug binds to the target.
Here is where supercomputers come in very handy,” Vorobyov said. He was awarded allocations on the Stampede2 system of the Texas Advanced Computing Center (TACC) by XSEDE, the Extreme Science and Engineering Discovery Environment funded by the National Science Foundation (NSF). XSEDE also provided supercomputing time on Comet at the San Diego Supercomputer Center, making use of Comet’s GPU and CPU nodes. The National Center for Supercomputing Applications allocated use of its NSF-funded Blue Waters system.
For long-term (greater than 5 microsecond) simulations of the relatively slow entry of the drug into cardiac cells, the scientists also made use of Anton 2 , a special-purpose supercomputer for biomolecular simulation designed and constructed by D. E. Shaw Research (DESRES) and hosted by Pittsburgh Supercomputing Center (PSC). Anton 2 is made available without cost by DESRES for non-commercial research use by US universities and other not-for-profit institutions, and is hosted by PSC with support from the NIH.
“Stampede 2 offered a large array of powerful multi-core CPU nodes, which we were able to efficiently use for dozens of molecular dynamics runs we had to do in parallel. Such efficiency and scalability rivaled and even exceeded other resources we used for those simulations including even GPU equipped nodes,” Vorobyov added.

University of California at Davis researchers Colleen Clancy (left) and Igor Vorobyov (right) co-authored the research on the computational pipeline for cardiotoxicity. Dr. Clancy is a professor and Vorobyov is an assistant professor, both at the Department of Physiology and Membrane Biology and the Department of Pharmacology at the UC Davis School of Medicine.
The team uses enhanced sampling simulations, called umbrella sampling, to facilitate the molecular dynamics simulations and yield quantitative determination of the binding affinities and rates of reaction needed for linking scales and feeding parameters further up the pipeline to the functional model.
“That was the novel linkage between our scales that we’ve both worked on for many years,” Clancy added. “But until now, there was no way to really connect those scales in a meaningful way.”
“This is a very novel link,” Vorobyov said, “because pretty much no one has done it before. We were able to successfully predict the outcome of the models. Just to take the parameters from the atomistic molecular dynamics simulation and predict how the cardiac cells and tissues respond to the drug application, we were able to predict the experimental prolongation of the QT interval.”
Clancy explained the key pieces of the study. “The first is connecting atomistic scale simulations to cardiac tissue simulations on the order of milliseconds, second, minutes That’s the first novelty,” she explained. “The second novelty is building a pipeline to predict cardiotoxicity, which hadn’t been done before. But the third piece is to move beyond QT interval as the surrogate, or proxy indicator for proarrhythmia.”
Source: Jorge Salazar at TACC





I had two heart attacks in 2012. About three years ago I learnt about HERBAL HEALTHPOINT and their successful herbal protocol for Coronary Artery Disease and Heart Failure, the herbal protocol is a miracle. Go to their web page w w w. herbalhealthpoint. c om. Its been years since this herbal treatment, my artery is clear and no sign of a heart attack. I do lots of walking and lost some weight too